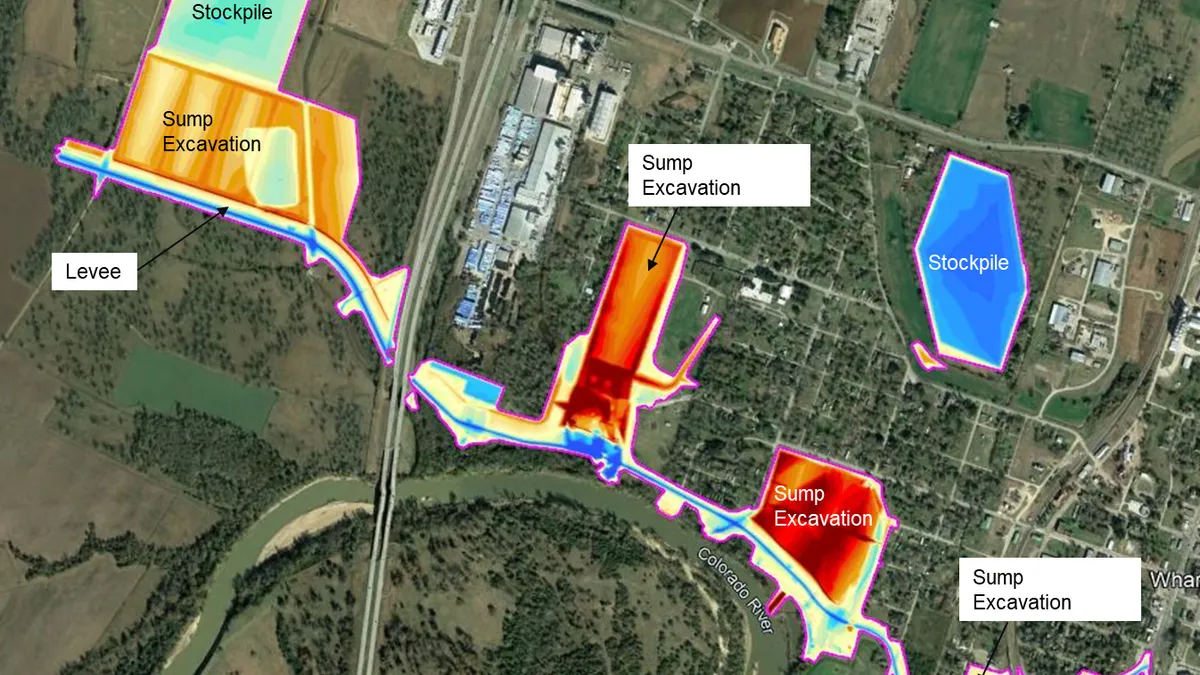
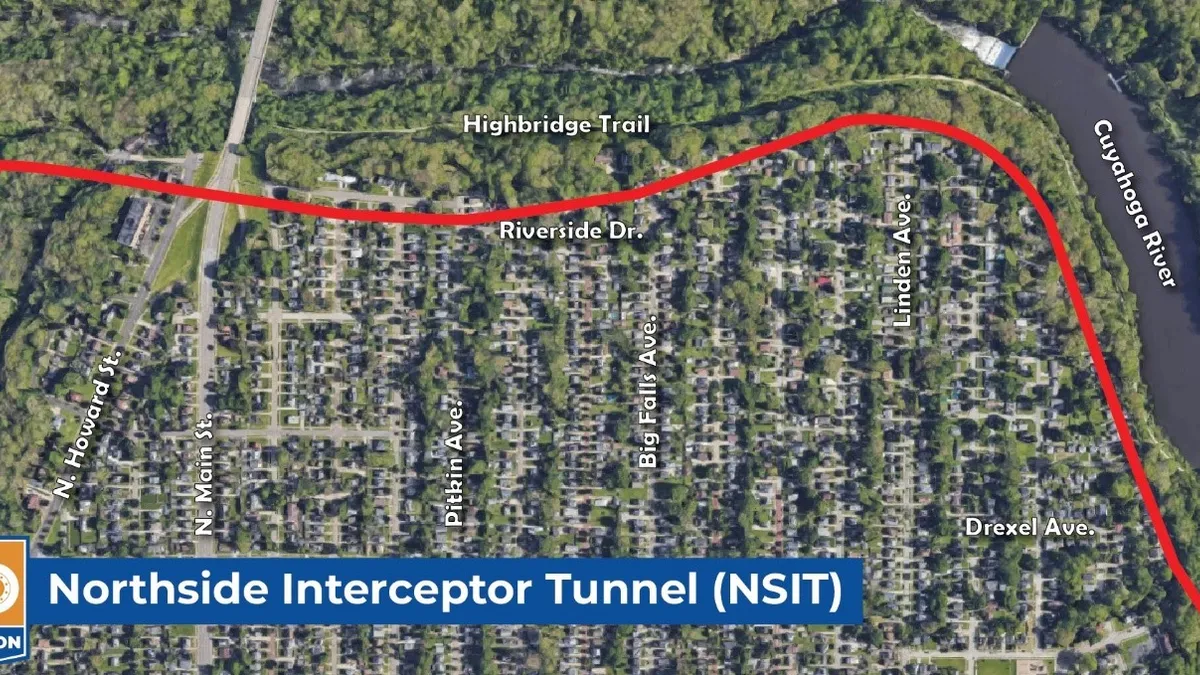
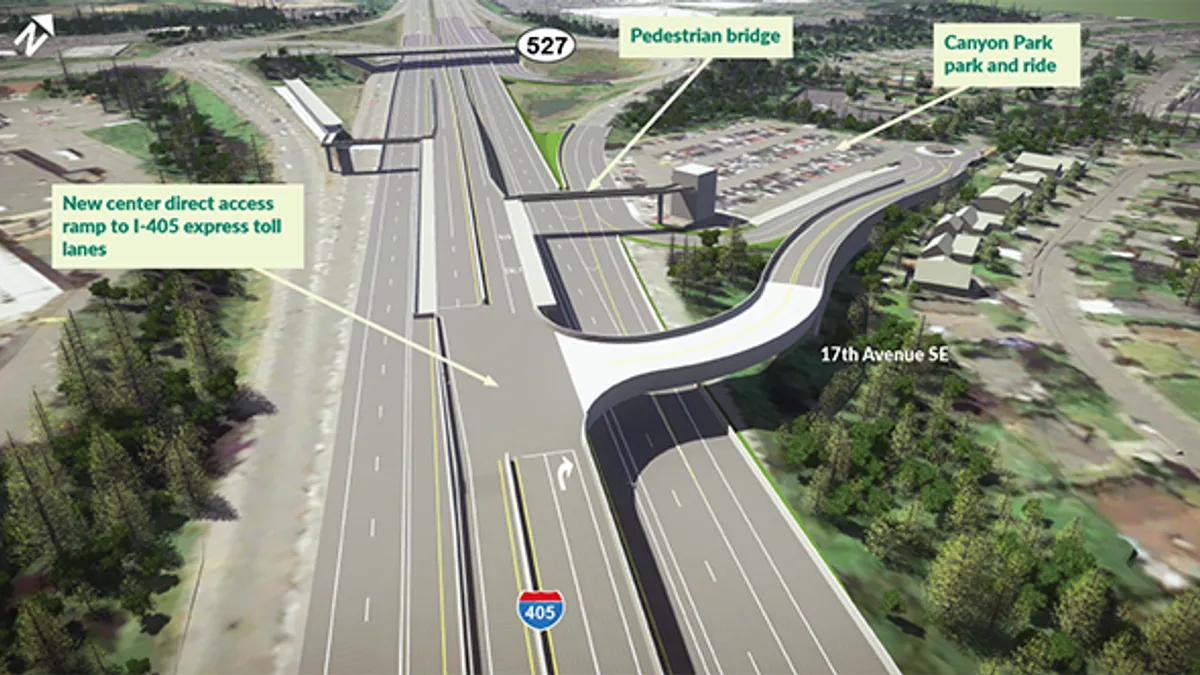
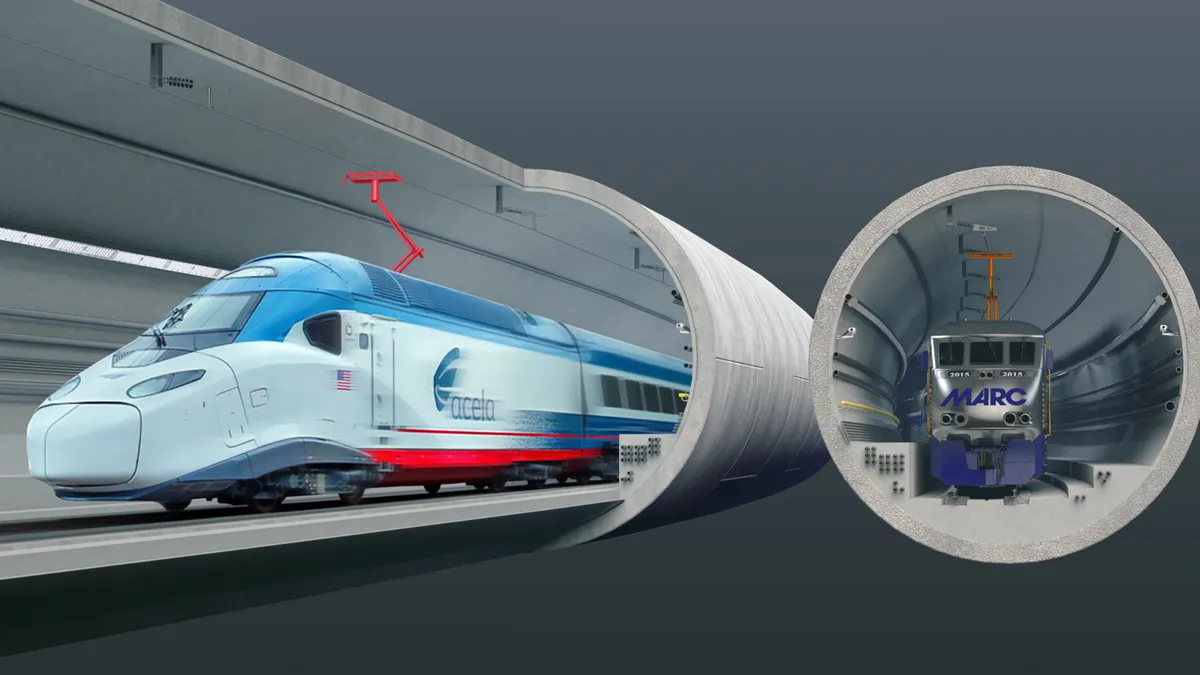
Dallas-based Jacobs recently secured a pivotal role in a major pharmaceutical project aimed at significantly improving global medical supply chains.
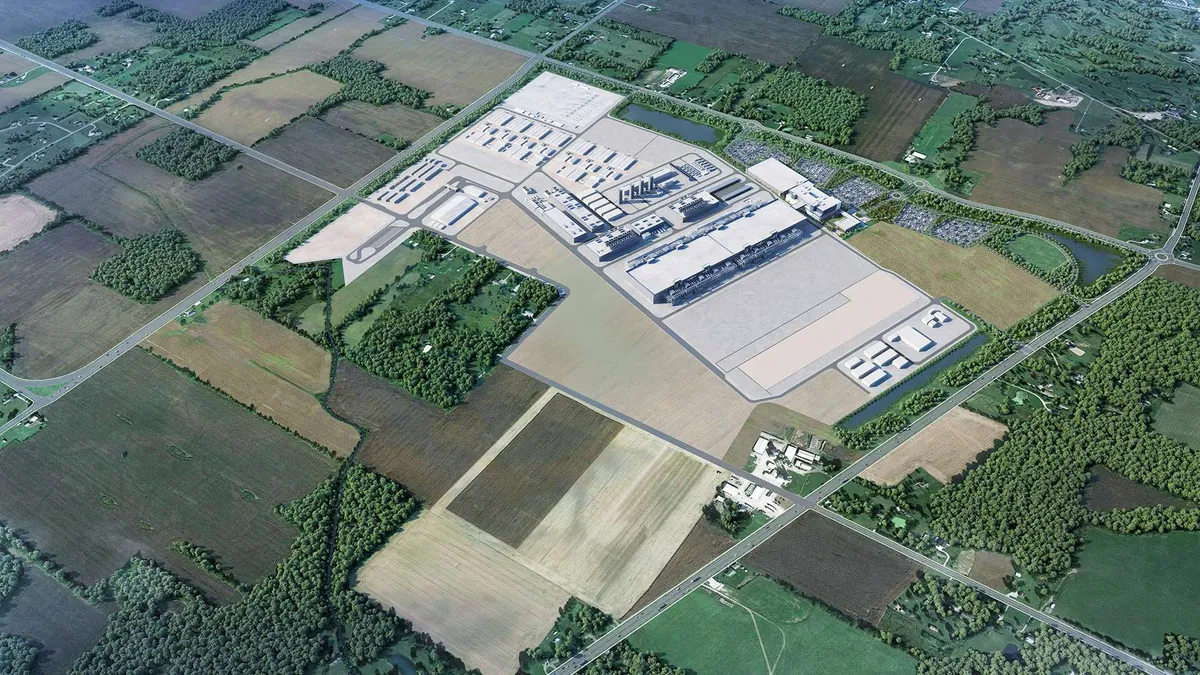
Fujifilm Diosynth Biotechnologies, a manufacturing organization for biologics, vaccines and therapies, selected Jacobs to support the delivery of a $1.2 billion expansion of its large-scale cell culture contract manufacturing site in Holly Springs, North Carolina, according to a news release.
The project, which builds on the site’s initial phase that broke ground in 2021, taps Jacobs to provide engineering, procurement and construction management services for the expansion. The development aims to increase manufacturing capacity with the addition of eight new 20,000-liter-cell culture bioreactors and about 400,000 square feet of manufacturing space. With the first phase now slated for completion in 2025, Jacobs said the additional expansion is slated to wrap up by 2028.
Koti Vadlamudi, Jacobs senior vice president, said the expansion leverages “digital innovation and modular design so that medicines and therapies are delivered with quality, flexibility, speed and consistency — now at an even larger scale,” according to a news release.
The project will significantly increase the biotechnology research company’s manufacturing capacity for biologic therapeutics and medicines. The facility will also provide comprehensive services including drug substance manufacturing, automated fill-finish and assembly, packaging and labeling services for global biopharmaceutical customers, said Kenneth Bilenberg, Fujifilm chief operating officer and executive vice president of operations.
Once completed, the Holly Springs site will be one of the largest end-to-end cell culture contract manufacturing facilities in North America, according to Fujifilm. The $1.2 billion investment forms part of Fujifilm’s broader $3.2 billion commitment to the site.